NEW CLARK CITY, Tarlac (January 30, 2026) – The Philippines’ dream of vaccine self-reliance is rapidly shifting from legislative paperwork to physical reality.
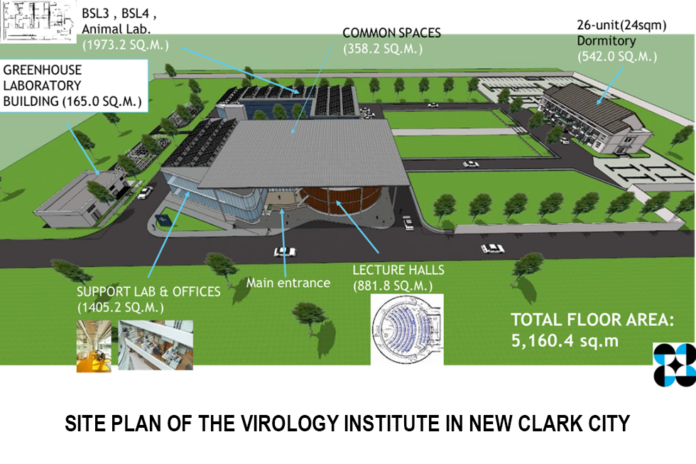
Construction is now officially underway for the Virology and Vaccine Institute of the Philippines (VIP) facility in New Clark City in Tarlac, marking a historic turning point in the nation’s public health infrastructure.
The physical rise of the Tarlac facility coincides with the completion of the final regulatory hurdles.
On January 28, the Department of Science and Technology (DOST) concluded its nationwide series of public consultations on the draft Implementing Rules and Regulations (IRR) of Republic Act 12290, also known as the VIP Act.
Signed into law by President Ferdinand Marcos Jr. on September 12, 2025, the VIP Act aims to end the country’s long-standing dependence on foreign-made vaccines.
While the measure underwent nearly three years of intense legislative debate, the current speed of construction in Tarlac signals a government eager to apply the lessons of the Covid-19 pandemic.
“Resilience is not merely surviving, but thriving,” stated DOST Secretary Renato Solidum Jr., emphasizing that the Tarlac institute is a long-term solution designed to ensure the country is never again left “at the back of the line” during a global health crisis.
The final leg of the consultations, held at the Sequoia Hotel in Parañaque City, gathered input from the Department of Health (DOH), UP Manila, and various civic groups.
These discussions focused on refining the “operating manual” for the institute, addressing concerns over bureaucratic redundancies and ensuring the facility can seamlessly collaborate with international researchers.
Once the IRR is finalized, it will provide the operational framework for the Tarlac site, which is envisioned as the premier hub for virology research and vaccine production in Southeast Asia.